Indian Council of Medical Research in collaboration with Bharat Biotech on friday launched the first indigenous vaccine to prevent Japanese encephalitis in children. So far, India used to import the vaccine from China. The Vero cell-derived purified inactivated Japanese encephalitis vaccine–JENVAC, which was approved by the Drug Controller General of India, pioneers as the first vaccine ever to be manufactured in a public-private collaboration. The vaccine aims to provide better immunogenicity and long term protection owing to unique manufacturing technologies.
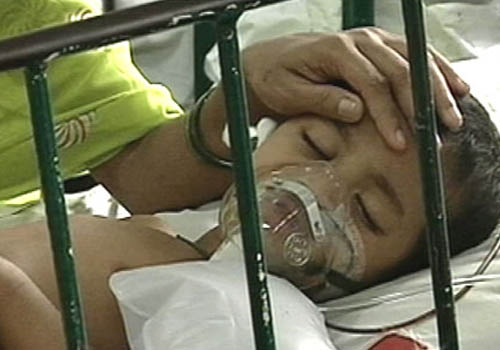
Japanese encephalitis is a mosquito-borne viral infection and is a very common cause of viral encephalitis in the eastern parts of Uttar Pradesh, where the disease affects close to 171 districts and claims hundreds of lives each year. Many children have ended up disabled because of this. The virus strain for this vaccine was isolated in Kolar (Karnataka), during the early 1980s and was characterized by National Institute of Virology based in Pune. The strains were then submitted to Bharat Biotech for further vaccine formulation.
The biggest advantage of JENVAC over live attenuated vaccines is that this vaccine could be administered even during an epidemic as it is an extremely purified and inactivated vaccine. Clinical trials proved JENVAC’s superlative performance as compared to a live vaccine. It achieved all its primary and secondary endpoints in the age-group of 1 to 50, following 1 to 2 doses of vaccination. Phase III trials revealed 98.7% sero-protection 28 days following the first dose, and 99.8% sero-protection 28 days following the second dosage. The results proved that JENVAC can be administered as a single dose at times of epidemics when mass vaccinations are required, and it can also be administered in 2-doses during routine immunisation as part of the National immunisation programme.
Bharat Biotech is scheming a dual pricing strategy for JENVAC, so as to cater to both public and private agencies. While the anticipated price is Rs 160/- per dose in private market, the government price is still in negotiation. Describing the vaccine as an “Indian solution for an Indian problem”, Union Health and Family Welfare Minister Ghulam Nabi Azad said this completely indigenous vaccine was an outstanding example of public-private partnership and a remarkable milestone in the emergence of India as an innovative and self-sufficient technology hub.
At present, the production capacity of JENVAC is 20mn doses, expandable to 60mn as per market needs.
Indian Council of Medical Research in collaboration with Bharat Biotech on friday launched the first indigenous vaccine to prevent Japanese encephalitis in children. So far, India used to import the vaccine from China. The Vero cell-derived purified inactivated Japanese encephalitis vaccine–JENVAC, which was approved by the Drug Controller General of India, pioneers as the first vaccine ever to be manufactured in a public-private collaboration. The vaccine aims to provide better immunogenicity and long term protection owing to unique manufacturing technologies.
Japanese encephalitis is a mosquito-borne viral infection and is a very common cause of viral encephalitis in the eastern parts of Uttar Pradesh, where the disease affects close to 171 districts and claims hundreds of lives each year. Many children have ended up disabled because of this. The virus strain for this vaccine was isolated in Kolar (Karnataka), during the early 1980s and was characterized by National Institute of Virology based in Pune. The strains were then submitted to Bharat Biotech for further vaccine formulation.
The biggest advantage of JENVAC over live attenuated vaccines is that this vaccine could be administered even during an epidemic as it is an extremely purified and inactivated vaccine. Clinical trials proved JENVAC’s superlative performance as compared to a live vaccine. It achieved all its primary and secondary endpoints in the age-group of 1 to 50, following 1 to 2 doses of vaccination. Phase III trials revealed 98.7% sero-protection 28 days following the first dose, and 99.8% sero-protection 28 days following the second dosage. The results proved that JENVAC can be administered as a single dose at times of epidemics when mass vaccinations are required, and it can also be administered in 2-doses during routine immunisation as part of the National immunisation programme.
Bharat Biotech is scheming a dual pricing strategy for JENVAC, so as to cater to both public and private agencies. While the anticipated price is Rs 160/- per dose in private market, the government price is still in negotiation. Describing the vaccine as an “Indian solution for an Indian problem”, Union Health and Family Welfare Minister Ghulam Nabi Azad said this completely indigenous vaccine was an outstanding example of public-private partnership and a remarkable milestone in the emergence of India as an innovative and self-sufficient technology hub.
At present, the production capacity of JENVAC is 20mn doses, expandable to 60mn as per market needs.